Abstract
Erythrocytes are produced rapidly in the bone-marrow of mammals, and undergo a series of cell divisions during development, culminating in the asymmetric separation of the future erythrocyte and its nucleus, in a process termed enucleation. Enucleation is the rate limiting step in the production of erythrocytes in vitro and our work aims to better understand enucleation to improve the production of RBCs ex vivo for transfusion medicine. Enucleation is thought to occur within the bone marrow in structures termed erythroblastic islands, which consist of a central macrophage surrounded by developing erythroblasts. Importantly, it is unclear if or how these erythroblastic islands modulate enucleation, and how they respond to various disease states like anaemia, bone marrow malignancies, or during stress erythropoiesis due to haemorrhage. To examine the dynamics and microenvironmental drivers of enucleation, we have conducted studies to identify the enucleation "niche" for erythroblasts within the bone marrow.
We have generated 3-dimensional images of optically clear mouse bone marrow using confocal and multiphoton microscopy to visualise and quantify in vivo sites of enucleation and the proximity of erythroblasts to the surrounding vascular sinuses and bone marrow resident macrophages. Using various molecular probes, we have been able to identify nascent reticulocytes to better understand where de novo RBCs are entering the circulation, and where circulating erythrocytes revisit the bone marrow. These findings, with the distinct morphological features and nuclear profiles of proerythroblasts (ProE), polychromatic erythroblasts (PolyE) and orthochromatic erythroblasts (OrthoE), have provided insight into the proximity of late-stage erythropoiesis to the vasculature, and where enucleation is occurring in situ; this has enabled quantification of the size and distribution of erythroid clusters. Our findings suggest there are various, dynamic sites of enucleation within the bone marrow, where clusters of erythroblasts enucleate at a range of proximities to erythroblastic island macrophages and the vasculature.
These experiments have been further complemented with analysis of erythropoietin receptor (EpoR) -driven nT/nG and mT/mG fluorescent reporter mice, which allow for visualisation of EpoR+ cells by nuclear GFP or membrane GFP respectively, in addition to colony stimulating factor 1 receptor (Csf1r)-driven GFP reporter mice to visualise the relationship between erythroblast clusters and macrophages, and to identify erythroblastic islands in situ. This work has culminated in the intravital imaging of mouse calvarium bone marrow, allowing visualisation of erythroblastic island structures and enucleation in real-time. To summarise, these studies provide novel insights into the dynamic nature and heterogeneity of erythroblastic islands, and the extrinsic factors regulating erythroid enucleation, in addition to defining the dynamic sites of erythroid enucleation in mouse bone marrow.
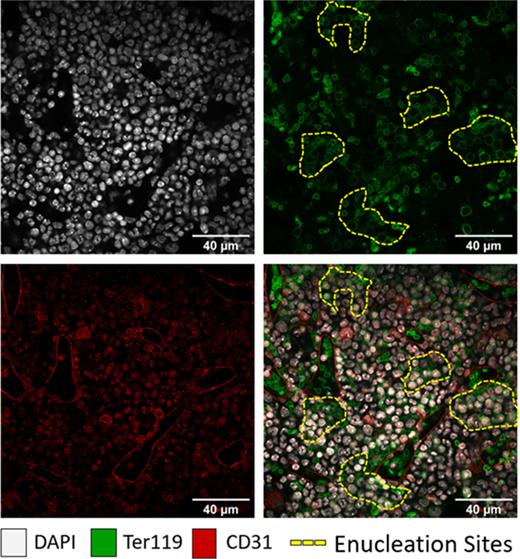
Figure 1. Examples of enucleation sites in mouse femur bone marrow.DAPI (white) and Ter119 (green) allow the visualisation of high-density regions of erythroblasts where enucleation occurs in proximity to CD31+ vascular sinuses (red).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal